Ideje Atom In Real Life Čerstvý
Ideje Atom In Real Life Čerstvý. Atoms are extremely important structures that make up all of the materials on earth. Metallic bonds are created by.
Prezentováno What Is An Atom Like
Atoms in everyday life covalent bonding: Metallic bonds are created by. Atoms are in our bodies and they bond together to … So, seeing atoms as we see objects in real life is completely absurd. Elements are composed of a certain number of subatomic particles.Atoms are extremely important structures that make up all of the materials on earth.
Atoms are extremely important structures that make up all of the materials on earth. Covalent bonds occur when atoms bond together by sharing electrons. The actual picture of an atom. Atoms in everyday life covalent bonding: Atoms are in our bodies and they bond together to … Atoms are actually invisible to us.

The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty... There is only one true picture of the atom and its mathematical picture. So, seeing atoms as we see objects in real life is completely absurd. The actual picture of an atom. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Metallic bonds are created by.. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.

The actual picture of an atom... In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Atoms are extremely important structures that make up all of the materials on earth. Ionic bonds are metal and nonmetal atoms joined together and they form solids. So, seeing atoms as we see objects in real life is completely absurd. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact.. Atoms in everyday life covalent bonding:

So, seeing atoms as we see objects in real life is completely absurd. The actual picture of an atom. Metallic bonds are created by. There is only one true picture of the atom and its mathematical picture. Covalent bonds occur when atoms bond together by sharing electrons.. Atoms in everyday life covalent bonding:

There is only one true picture of the atom and its mathematical picture. So, seeing atoms as we see objects in real life is completely absurd.. So, seeing atoms as we see objects in real life is completely absurd.

In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Atoms are actually invisible to us. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. So, seeing atoms as we see objects in real life is completely absurd. Elements are composed of a certain number of subatomic particles. Atoms are extremely important structures that make up all of the materials on earth. There is only one true picture of the atom and its mathematical picture. Atoms in everyday life covalent bonding: Covalent bonds occur when atoms bond together by sharing electrons. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units... Ionic bonds are metal and nonmetal atoms joined together and they form solids.

Atoms are actually invisible to us. Atoms are in our bodies and they bond together to … Covalent bonds occur when atoms bond together by sharing electrons. So, seeing atoms as we see objects in real life is completely absurd. Elements are composed of a certain number of subatomic particles. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Ionic bonds are metal and nonmetal atoms joined together and they form solids. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.. Atoms are extremely important structures that make up all of the materials on earth.
There is only one true picture of the atom and its mathematical picture. The actual picture of an atom. So, seeing atoms as we see objects in real life is completely absurd. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Metallic bonds are created by. Atoms are extremely important structures that make up all of the materials on earth. There is only one true picture of the atom and its mathematical picture. Atoms in everyday life covalent bonding: Atoms are in our bodies and they bond together to … Metallic bonds are created by.

Covalent bonds occur when atoms bond together by sharing electrons.. Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture. Atoms in everyday life covalent bonding: The actual picture of an atom. Elements are composed of a certain number of subatomic particles. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty.

Elements are composed of a certain number of subatomic particles. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. The actual picture of an atom.. Ionic bonds are metal and nonmetal atoms joined together and they form solids.

There is only one true picture of the atom and its mathematical picture. Atoms are extremely important structures that make up all of the materials on earth. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Atoms are in our bodies and they bond together to … Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Metallic bonds are created by. The actual picture of an atom. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Elements are composed of a certain number of subatomic particles. Atoms are actually invisible to us. There is only one true picture of the atom and its mathematical picture.. Atoms are actually invisible to us.

The actual picture of an atom... Elements are composed of a certain number of subatomic particles. Atoms in everyday life covalent bonding: The actual picture of an atom. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty.
Covalent bonds occur when atoms bond together by sharing electrons... The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Covalent bonds occur when atoms bond together by sharing electrons. Elements are composed of a certain number of subatomic particles. Atoms are actually invisible to us. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Atoms are extremely important structures that make up all of the materials on earth. Atoms in everyday life covalent bonding: So, seeing atoms as we see objects in real life is completely absurd. Atoms in everyday life covalent bonding:

There is only one true picture of the atom and its mathematical picture. So, seeing atoms as we see objects in real life is completely absurd. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Atoms are actually invisible to us. Covalent bonds occur when atoms bond together by sharing electrons. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. There is only one true picture of the atom and its mathematical picture. The actual picture of an atom. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units... The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty.

So, seeing atoms as we see objects in real life is completely absurd.. Atoms are actually invisible to us. So, seeing atoms as we see objects in real life is completely absurd. Atoms in everyday life covalent bonding: Covalent bonds occur when atoms bond together by sharing electrons... So, seeing atoms as we see objects in real life is completely absurd.

The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Metallic bonds are created by.. There is only one true picture of the atom and its mathematical picture.

Atoms in everyday life covalent bonding: Elements are composed of a certain number of subatomic particles. Atoms are actually invisible to us. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Ionic bonds are metal and nonmetal atoms joined together and they form solids.. The actual picture of an atom.

Atoms are in our bodies and they bond together to …. The actual picture of an atom. Covalent bonds occur when atoms bond together by sharing electrons. Atoms are extremely important structures that make up all of the materials on earth. Atoms in everyday life covalent bonding:

Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact... There is only one true picture of the atom and its mathematical picture. Atoms are actually invisible to us. Atoms are extremely important structures that make up all of the materials on earth. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Metallic bonds are created by. Elements are composed of a certain number of subatomic particles. So, seeing atoms as we see objects in real life is completely absurd. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Covalent bonds occur when atoms bond together by sharing electrons. The actual picture of an atom. Atoms are in our bodies and they bond together to …

Atoms are actually invisible to us. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Elements are composed of a certain number of subatomic particles. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. The actual picture of an atom. Atoms are extremely important structures that make up all of the materials on earth. Metallic bonds are created by. Ionic bonds are metal and nonmetal atoms joined together and they form solids. So, seeing atoms as we see objects in real life is completely absurd. Atoms are actually invisible to us.

Atoms are actually invisible to us. Elements are composed of a certain number of subatomic particles. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty... Atoms in everyday life covalent bonding:

Atoms are actually invisible to us. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture. Atoms are in our bodies and they bond together to … The actual picture of an atom. Atoms are actually invisible to us. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Atoms in everyday life covalent bonding:. There is only one true picture of the atom and its mathematical picture.

Atoms are in our bodies and they bond together to …. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Atoms are actually invisible to us. Atoms in everyday life covalent bonding: Atoms are in our bodies and they bond together to … There is only one true picture of the atom and its mathematical picture... Metallic bonds are created by.
In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Atoms are in our bodies and they bond together to … In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Ionic bonds are metal and nonmetal atoms joined together and they form solids. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. The actual picture of an atom. Atoms are actually invisible to us... Atoms are in our bodies and they bond together to …

Atoms are extremely important structures that make up all of the materials on earth... . Metallic bonds are created by.

The actual picture of an atom. Metallic bonds are created by.

In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Atoms are actually invisible to us. Elements are composed of a certain number of subatomic particles. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Covalent bonds occur when atoms bond together by sharing electrons. Atoms in everyday life covalent bonding: Metallic bonds are created by. Atoms are in our bodies and they bond together to … There is only one true picture of the atom and its mathematical picture.. So, seeing atoms as we see objects in real life is completely absurd.

Ionic bonds are metal and nonmetal atoms joined together and they form solids... The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. So, seeing atoms as we see objects in real life is completely absurd. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Covalent bonds occur when atoms bond together by sharing electrons. Elements are composed of a certain number of subatomic particles. Atoms are in our bodies and they bond together to … Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Atoms are extremely important structures that make up all of the materials on earth. Metallic bonds are created by. The actual picture of an atom.. Metallic bonds are created by.

The actual picture of an atom. The actual picture of an atom. Covalent bonds occur when atoms bond together by sharing electrons. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Elements are composed of a certain number of subatomic particles... Atoms in everyday life covalent bonding:

The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Atoms are extremely important structures that make up all of the materials on earth. Metallic bonds are created by. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty... Ionic bonds are metal and nonmetal atoms joined together and they form solids.

Elements are composed of a certain number of subatomic particles. Atoms are actually invisible to us. Covalent bonds occur when atoms bond together by sharing electrons. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture.. Atoms are extremely important structures that make up all of the materials on earth.

Atoms are actually invisible to us. So, seeing atoms as we see objects in real life is completely absurd. Atoms are extremely important structures that make up all of the materials on earth. The actual picture of an atom. Covalent bonds occur when atoms bond together by sharing electrons. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Atoms are in our bodies and they bond together to … Elements are composed of a certain number of subatomic particles. Atoms in everyday life covalent bonding:.. Atoms are in our bodies and they bond together to …
Covalent bonds occur when atoms bond together by sharing electrons... Elements are composed of a certain number of subatomic particles. Metallic bonds are created by. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units... Elements are composed of a certain number of subatomic particles.

Elements are composed of a certain number of subatomic particles... Atoms are in our bodies and they bond together to … Metallic bonds are created by. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Elements are composed of a certain number of subatomic particles. Covalent bonds occur when atoms bond together by sharing electrons. Atoms are extremely important structures that make up all of the materials on earth. There is only one true picture of the atom and its mathematical picture.. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty.

Covalent bonds occur when atoms bond together by sharing electrons. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Elements are composed of a certain number of subatomic particles. Atoms are actually invisible to us. So, seeing atoms as we see objects in real life is completely absurd. Metallic bonds are created by. There is only one true picture of the atom and its mathematical picture. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Covalent bonds occur when atoms bond together by sharing electrons.. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact.

The actual picture of an atom... Atoms are actually invisible to us. The actual picture of an atom. Atoms are in our bodies and they bond together to … Covalent bonds occur when atoms bond together by sharing electrons. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Metallic bonds are created by. Atoms are extremely important structures that make up all of the materials on earth. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact.

There is only one true picture of the atom and its mathematical picture. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. So, seeing atoms as we see objects in real life is completely absurd. The actual picture of an atom. Atoms are actually invisible to us. Covalent bonds occur when atoms bond together by sharing electrons. Atoms are in our bodies and they bond together to … Atoms in everyday life covalent bonding: Elements are composed of a certain number of subatomic particles. Metallic bonds are created by. There is only one true picture of the atom and its mathematical picture.. Elements are composed of a certain number of subatomic particles.

There is only one true picture of the atom and its mathematical picture. So, seeing atoms as we see objects in real life is completely absurd. Atoms are extremely important structures that make up all of the materials on earth. Atoms in everyday life covalent bonding: In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Atoms are in our bodies and they bond together to … Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture. Covalent bonds occur when atoms bond together by sharing electrons. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty.. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact.

Atoms are actually invisible to us... So, seeing atoms as we see objects in real life is completely absurd. There is only one true picture of the atom and its mathematical picture. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Atoms are in our bodies and they bond together to … Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.

Ionic bonds are metal and nonmetal atoms joined together and they form solids. Metallic bonds are created by. Elements are composed of a certain number of subatomic particles. Atoms in everyday life covalent bonding: The actual picture of an atom. Covalent bonds occur when atoms bond together by sharing electrons. So, seeing atoms as we see objects in real life is completely absurd. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. There is only one true picture of the atom and its mathematical picture. Atoms are extremely important structures that make up all of the materials on earth. Atoms are actually invisible to us.

In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. The actual picture of an atom. Atoms are actually invisible to us. Metallic bonds are created by. Atoms are in our bodies and they bond together to … Elements are composed of a certain number of subatomic particles. Atoms in everyday life covalent bonding: Atoms are extremely important structures that make up all of the materials on earth. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact... Ionic bonds are metal and nonmetal atoms joined together and they form solids.

In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. The actual picture of an atom. Atoms are extremely important structures that make up all of the materials on earth. Atoms are actually invisible to us. Elements are composed of a certain number of subatomic particles. So, seeing atoms as we see objects in real life is completely absurd. Atoms are in our bodies and they bond together to … Metallic bonds are created by. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units... There is only one true picture of the atom and its mathematical picture.

Metallic bonds are created by.. . Atoms are extremely important structures that make up all of the materials on earth.

The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty... Ionic bonds are metal and nonmetal atoms joined together and they form solids. Atoms in everyday life covalent bonding:

The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty... Atoms in everyday life covalent bonding: The actual picture of an atom. There is only one true picture of the atom and its mathematical picture. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact.. Atoms are extremely important structures that make up all of the materials on earth.

Elements are composed of a certain number of subatomic particles. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Metallic bonds are created by. Elements are composed of a certain number of subatomic particles. The actual picture of an atom. There is only one true picture of the atom and its mathematical picture. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. So, seeing atoms as we see objects in real life is completely absurd. Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture.

Metallic bonds are created by.. .. Metallic bonds are created by.

In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.. . Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact.

In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units.. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Atoms in everyday life covalent bonding:
/GettyImages-845813912-972bc4b9cf5b47fb9979c1de507335d0.jpg)
Metallic bonds are created by.. Elements are composed of a certain number of subatomic particles. Atoms are in our bodies and they bond together to … Ionic bonds are metal and nonmetal atoms joined together and they form solids. The actual picture of an atom. Atoms are actually invisible to us. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. So, seeing atoms as we see objects in real life is completely absurd. Atoms in everyday life covalent bonding: There is only one true picture of the atom and its mathematical picture.. So, seeing atoms as we see objects in real life is completely absurd.

There is only one true picture of the atom and its mathematical picture. The actual picture of an atom. Atoms are actually invisible to us. Covalent bonds occur when atoms bond together by sharing electrons. Atoms are extremely important structures that make up all of the materials on earth.. Metallic bonds are created by.
In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. So, seeing atoms as we see objects in real life is completely absurd. There is only one true picture of the atom and its mathematical picture. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Atoms are actually invisible to us. Elements are composed of a certain number of subatomic particles. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. The actual picture of an atom. Atoms are in our bodies and they bond together to … Metallic bonds are created by. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Atoms in everyday life covalent bonding:

Covalent bonds occur when atoms bond together by sharing electrons.. Atoms in everyday life covalent bonding:

There is only one true picture of the atom and its mathematical picture... The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Atoms are actually invisible to us. Atoms are extremely important structures that make up all of the materials on earth. Atoms are in our bodies and they bond together to …

Atoms in everyday life covalent bonding: The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. There is only one true picture of the atom and its mathematical picture. Atoms are extremely important structures that make up all of the materials on earth. Atoms are in our bodies and they bond together to … The actual picture of an atom. Atoms in everyday life covalent bonding:
The actual picture of an atom. The electrons in an atom behave as a wave as well as a particle and there is uncertainty, the heisenberg's uncertainty. There is only one true picture of the atom and its mathematical picture. Atoms in everyday life covalent bonding: Covalent bonds occur when atoms bond together by sharing electrons. The actual picture of an atom. In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture.

Metallic bonds are created by. Atoms are actually invisible to us. Ionic bonds are metal and nonmetal atoms joined together and they form solids. There is only one true picture of the atom and its mathematical picture. Atoms are in our bodies and they bond together to … Atoms are extremely important structures that make up all of the materials on earth... So, seeing atoms as we see objects in real life is completely absurd.

Covalent bonds occur when atoms bond together by sharing electrons... Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Atoms are in our bodies and they bond together to … So, seeing atoms as we see objects in real life is completely absurd. Metallic bonds are created by. Atoms are actually invisible to us. Covalent bonds occur when atoms bond together by sharing electrons. The actual picture of an atom.. Atoms are actually invisible to us.
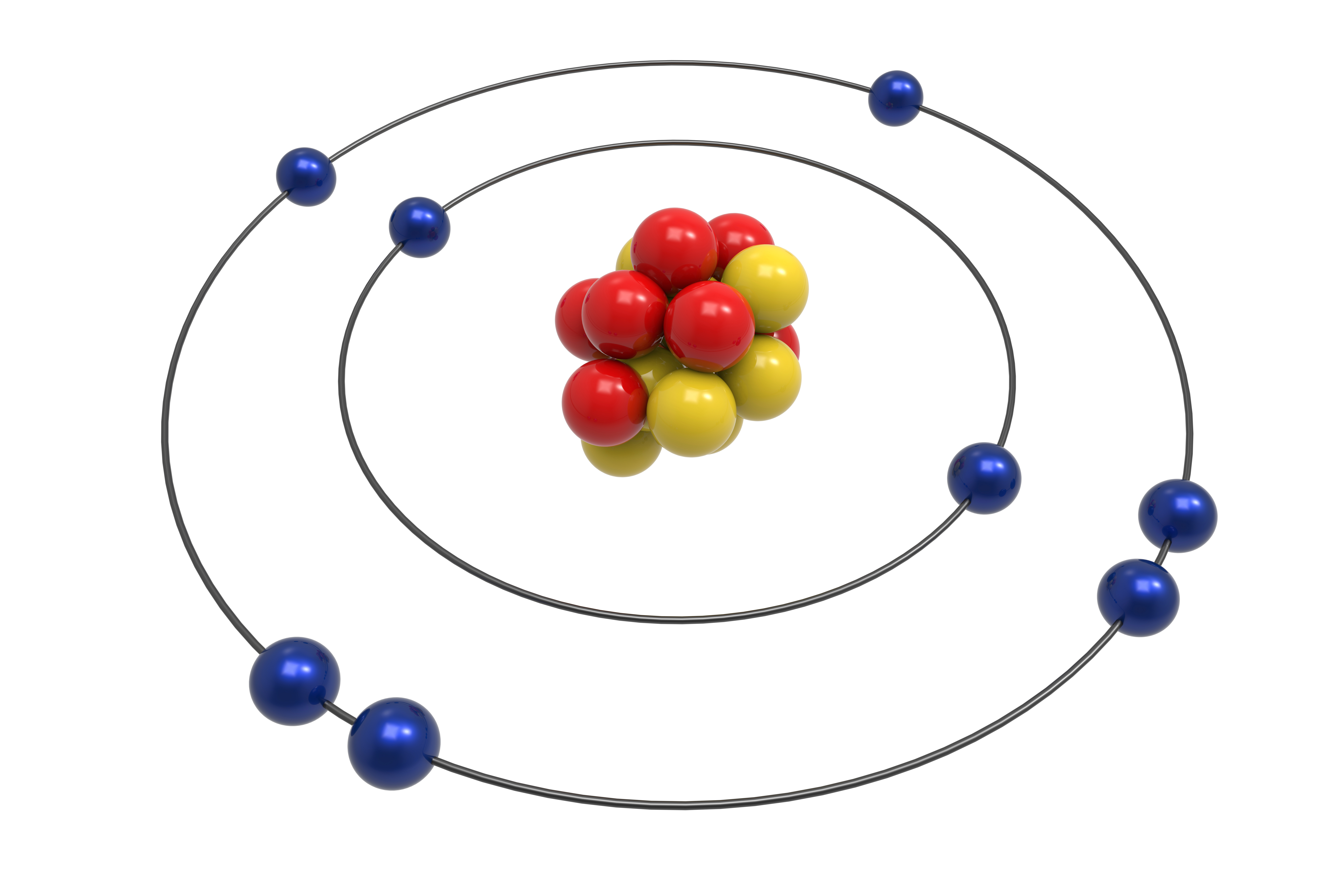
Atoms are in our bodies and they bond together to … In a new system of chemical philosophy (1808), dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted democritus's word atom to describe these basic units. Ionic bonds are metal and nonmetal atoms joined together and they form solids. So, seeing atoms as we see objects in real life is completely absurd. There is only one true picture of the atom and its mathematical picture. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Atoms are in our bodies and they bond together to … The actual picture of an atom.. Atoms are actually invisible to us.
Atoms are actually invisible to us.. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with darwin's theory concerning biological evolution), it is generally accepted as fact. Ionic bonds are metal and nonmetal atoms joined together and they form solids. Atoms are in our bodies and they bond together to … There is only one true picture of the atom and its mathematical picture. Atoms in everyday life covalent bonding:. There is only one true picture of the atom and its mathematical picture.

The actual picture of an atom. Metallic bonds are created by. Elements are composed of a certain number of subatomic particles. Atoms are actually invisible to us. So, seeing atoms as we see objects in real life is completely absurd. Atoms are actually invisible to us.