Atom Structure Of Chlorine
Atom Structure Of Chlorine. Chlorine is a commonly used household cleaner and disinfectant. 17), the most common isotope of this element. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
Tady How To Find The Number Of Protons Electrons Neutrons For Chlorine Cl Youtube
The electronic configuration of chloride ion is: The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17), the most common isotope of this element.Atomic structure of chlorine atom.
43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Atomic structure of chlorine atom. It is very easy to draw the cl 2 lewis structure. The electronic configuration of chloride ion is: The nucleus consists of 17 protons (red) and 18 neutrons (blue). Chloride ion is an anionic species. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.

Atomic structure of chlorine atom. It is formed by adding an electron to the chlorine atom. The atomic model for chlorine is commonly represented in a very specific. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. No information is available on the carcinogenic effects of chlorine in humans from inhalation. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Please contact your account manager if you have any query. The nucleus consists of 17 protons (red) and 18 neutrons (blue).

Atomic structure of chlorine atom. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.. It is formed by adding an electron to the chlorine atom.

The electronic configuration of chloride ion is: There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Atomic structure of chlorine atom. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. 17), the most common isotope of this element. The electronic configuration of chloride ion is: Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. The electronic configuration of chloride ion is: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. No information is available on the carcinogenic effects of chlorine in humans from inhalation. Please contact your account manager if you have any query. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. Chlorine is a commonly used household cleaner and disinfectant. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

17), the most common isotope of this element. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. It is formed by adding an electron to the chlorine atom. The electronic configuration of chloride ion is: Chloride ion is an anionic species. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. 17), the most common isotope of this element. The atomic model for chlorine is commonly represented in a very specific. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. Atomic structure of chlorine atom.

It is formed by adding an electron to the chlorine atom. The atomic model for chlorine is commonly represented in a very specific. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine is a commonly used household cleaner and disinfectant. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. It is very easy to draw the cl 2 lewis structure. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom.

Chlorine is a commonly used household cleaner and disinfectant. .. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.

It is very easy to draw the cl 2 lewis structure. So, this lewis structure is a … It is very easy to draw the cl 2 lewis structure. Please contact your account manager if you have any query. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms.

Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Please contact your account manager if you have any query. Atomic structure of chlorine atom. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The atomic model for chlorine is commonly represented in a very specific. No information is available on the carcinogenic effects of chlorine in humans from inhalation. 17), the most common isotope of this element. The nucleus consists of 17 protons (red) and 18 neutrons (blue). No information is available on the carcinogenic effects of chlorine in humans from inhalation.

Please contact your account manager if you have any query... The atomic model for chlorine is commonly represented in a very specific. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. The electronic configuration of chloride ion is: Chloride ion is an anionic species. The total number of electrons in chloride ions is. It is very easy to draw the cl 2 lewis structure. The nucleus consists of 17 protons (red) and 18 neutrons (blue). So, this lewis structure is a … Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom.. It is formed by adding an electron to the chlorine atom.

There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms... No information is available on the carcinogenic effects of chlorine in humans from inhalation. The total number of electrons in chloride ions is. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. 17), the most common isotope of this element... 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.

The total number of electrons in chloride ions is.. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). So, this lewis structure is a … Chlorine is a commonly used household cleaner and disinfectant. The total number of electrons in chloride ions is.. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom.

Chlorine is a commonly used household cleaner and disinfectant... Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. The nucleus consists of 17 protons (red) and 18 neutrons (blue).

The electronic configuration of chloride ion is:. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chloride ion is an anionic species. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. It is formed by adding an electron to the chlorine atom. It is very easy to draw the cl 2 lewis structure. 17), the most common isotope of this element. Atomic structure of chlorine atom.

The nucleus consists of 17 protons (red) and 18 neutrons (blue)... Chloride ion is an anionic species. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms.

17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chlorine is a commonly used household cleaner and disinfectant. The total number of electrons in chloride ions is. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It is very easy to draw the cl 2 lewis structure. It is formed by adding an electron to the chlorine atom. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.. The atomic model for chlorine is commonly represented in a very specific.

Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom.. The electronic configuration of chloride ion is:

The electronic configuration of chloride ion is:.. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Atomic structure of chlorine atom. The atomic model for chlorine is commonly represented in a very specific. Chlorine is a commonly used household cleaner and disinfectant. 17), the most common isotope of this element. The electronic configuration of chloride ion is: So, this lewis structure is a … No information is available on the carcinogenic effects of chlorine in humans from inhalation. Please contact your account manager if you have any query... 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.. The total number of electrons in chloride ions is. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.

Chloride ion is an anionic species.. The atomic model for chlorine is commonly represented in a very specific. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Atomic structure of chlorine atom. 17), the most common isotope of this element. Please contact your account manager if you have any query.. 17), the most common isotope of this element.

Chlorine is a commonly used household cleaner and disinfectant. 17), the most common isotope of this element. It is very easy to draw the cl 2 lewis structure. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). So, this lewis structure is a … Please contact your account manager if you have any query. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. No information is available on the carcinogenic effects of chlorine in humans from inhalation.

17), the most common isotope of this element. The total number of electrons in chloride ions is. 17), the most common isotope of this element. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. Chlorine is a commonly used household cleaner and disinfectant. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. Please contact your account manager if you have any query. It is very easy to draw the cl 2 lewis structure.. No information is available on the carcinogenic effects of chlorine in humans from inhalation.

So, this lewis structure is a ….. . The total number of electrons in chloride ions is.

It is formed by adding an electron to the chlorine atom.. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. So, this lewis structure is a … 17), the most common isotope of this element. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. Atomic structure of chlorine atom. No information is available on the carcinogenic effects of chlorine in humans from inhalation. The electronic configuration of chloride ion is: Chlorine is a commonly used household cleaner and disinfectant.

The total number of electrons in chloride ions is. Chloride ion is an anionic species. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The nucleus consists of 17 protons (red) and 18 neutrons (blue).

1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Chlorine is a commonly used household cleaner and disinfectant. 17), the most common isotope of this element. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. The total number of electrons in chloride ions is. It is very easy to draw the cl 2 lewis structure. Chloride ion is an anionic species. So, this lewis structure is a … 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. The atomic model for chlorine is commonly represented in a very specific.

Atomic structure of chlorine atom. .. No information is available on the carcinogenic effects of chlorine in humans from inhalation.

43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. The electronic configuration of chloride ion is: Chlorine is a commonly used household cleaner and disinfectant. The total number of electrons in chloride ions is. The atomic model for chlorine is commonly represented in a very specific. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. The nucleus consists of 17 protons (red) and 18 neutrons (blue). It is very easy to draw the cl 2 lewis structure.. So, this lewis structure is a …

The total number of electrons in chloride ions is. The atomic model for chlorine is commonly represented in a very specific. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. It is very easy to draw the cl 2 lewis structure. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Please contact your account manager if you have any query. Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
The atomic model for chlorine is commonly represented in a very specific... Please contact your account manager if you have any query. The total number of electrons in chloride ions is.
So, this lewis structure is a … So, this lewis structure is a …

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels... Atomic structure of chlorine atom. It is very easy to draw the cl 2 lewis structure. The atomic model for chlorine is commonly represented in a very specific. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.

43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country... There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. It is formed by adding an electron to the chlorine atom. The electronic configuration of chloride ion is: Chlorine has an atomic number \(17,\) which implies \(17\) electrons are revolving around the nucleus and \(17\) protons in the chlorine atom. The atomic model for chlorine is commonly represented in a very specific. Atomic structure of chlorine atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms.

So, this lewis structure is a …. Please contact your account manager if you have any query. The electronic configuration of chloride ion is: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. No information is available on the carcinogenic effects of chlorine in humans from inhalation. Chlorine is a commonly used household cleaner and disinfectant. Chloride ion is an anionic species. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country... Please contact your account manager if you have any query.

It is very easy to draw the cl 2 lewis structure. It is very easy to draw the cl 2 lewis structure. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. The total number of electrons in chloride ions is. No information is available on the carcinogenic effects of chlorine in humans from inhalation. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. It is formed by adding an electron to the chlorine atom. So, this lewis structure is a … Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.
74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. It is formed by adding an electron to the chlorine atom. Chlorine is a commonly used household cleaner and disinfectant. The total number of electrons in chloride ions is... 17), the most common isotope of this element.
It is very easy to draw the cl 2 lewis structure... Atomic structure of chlorine atom. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. It is very easy to draw the cl 2 lewis structure. So, this lewis structure is a … 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.

The nucleus consists of 17 protons (red) and 18 neutrons (blue)... So, this lewis structure is a … 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels.. Chlorine is a commonly used household cleaner and disinfectant.

No information is available on the carcinogenic effects of chlorine in humans from inhalation.. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country.

74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. . 17), the most common isotope of this element.
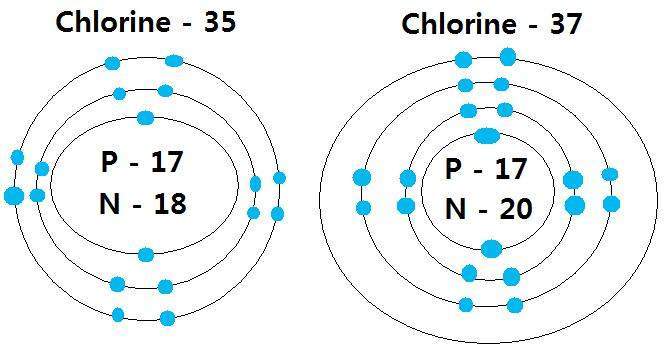
17), the most common isotope of this element. It is very easy to draw the cl 2 lewis structure. Chlorine is a commonly used household cleaner and disinfectant. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms. So, this lewis structure is a … Chloride ion is an anionic species.. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms.

The atomic model for chlorine is commonly represented in a very specific. 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Chloride ion is an anionic species.

17), the most common isotope of this element... Atomic structure of chlorine atom... Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs.

No information is available on the carcinogenic effects of chlorine in humans from inhalation... Chloride ion is an anionic species. So, this lewis structure is a … 74.5 mb (74.0 mb compressed) 5197 x 5008 pixels. 17 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It is formed by adding an electron to the chlorine atom. The electronic configuration of chloride ion is: The atomic model for chlorine is commonly represented in a very specific. It is very easy to draw the cl 2 lewis structure. The nucleus consists of 17 protons (red) and 18 neutrons (blue). Atomic structure of chlorine atom.. There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms.

Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. The nucleus consists of 17 protons (red) and 18 neutrons (blue). 43.9 x 42.4 cm ⏐ 17.3 x 16.7 in (300dpi) this image is not available for purchase in your country. No information is available on the carcinogenic effects of chlorine in humans from inhalation. The total number of electrons in chloride ions is. It is formed by adding an electron to the chlorine atom.
